Water and a few drops of blue food coloring (dye) were added to a pot of water on a hot plate. The solution was allowed to come to a boil, which is assumed to be vapor liquid equilibrium. Steam rising above the solution collects on the lid being held above the pot. The condensation is then allowed to collect in a beaker.
Notice that the condensation collecting on the lid of the pot and in the beaker is clear; it doesn't appear blue like the water in the pot. Below is a picture of the condensation that was collected in the beaker.

Why is it that the condensation appears clear and not blue?
We are assuming that this process is like a one step distillation. The food coloring is a much larger molecule that water and will therefore have a higher boiling point.
The assume the graph below represents the vapor-liquid equilibrium of dye in water. Our solution has only a few drops of dye compared to several hundred milliliters of water. Therefore, the mole fraction of dye in the liquid phase (x) is very small (less than 0.01). Therefore, the corresponding mole fraction of dye in the vapor phase (y) is even smaller.
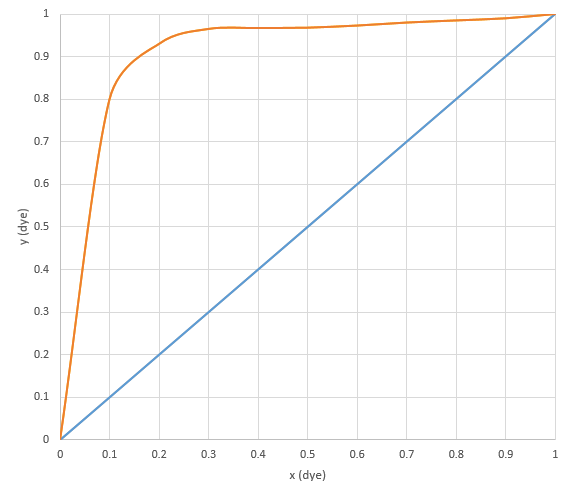
Because of the extremely small presence of dye in the vapor phase, the condensation, with almost no dye in it, appears to be clear.

This work is licensed under a Creative Commons Attribution 4.0 International License.
Template by OS Templates